Medsafe blocks sales of Covid-19 'treatment' Artemisia annua

Medsafe has won praise for moving quickly to stop sales of a herbal remedy linked to Covid-19.

Artemisia Spp. Derivatives for COVID-19 Treatment: Anecdotal Use, Political Hype, Treatment Potential, Challenges, and Road Map to Randomized Clinical Trials in: The American Journal of Tropical Medicine and Hygiene Volume 103 Issue 3 (2020)

Artemisinin (Sweet Wormwood Cleanse)(Artemisia Annua) 200mg Per Serving, 120 Capsules (Two Month Supply) Vegan Safe, Non-GMO, Gluten Free, Manufactured in The USA (Immune Support) by Double Wood : Health & Household

Arthritis drugs found to reduce risk of death in severe Covid-19 cases

Network pharmacology-based predictions of active components and pharmacological mechanisms of Artemisia annua L. for the treatment of the novel Corona virus disease 2019 (COVID-19), BMC Complementary Medicine and Therapies
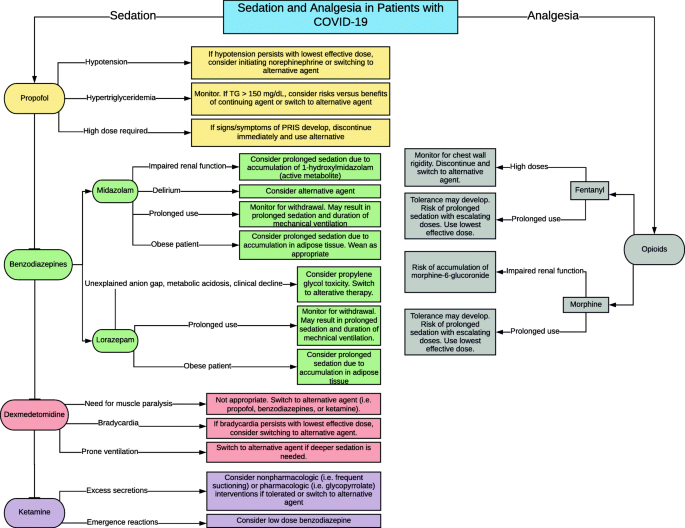
Challenges in Sedation Management in Critically Ill Patients with COVID-19: a Brief Review

COVID-19 Lockdown Exit Analysis

Medsafe blocks sales of COVID-19 'treatment' Artemisia annua extract

COVID-19 Lockdown Exit Analysis

Cost-Utility and Cost-Effectiveness Analysis of disease-modifying drugs of Relapsing-Remitting Multiple Sclerosis: A Systematic Review

Medsafe blocks sales of COVID-19 'treatment' Artemisia annua extract