Lista de encabezados
thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange

Descripción
In my chemistry teacher's notes, some notations concerning the heat $Q$ are marked as inappropriate. $Q$: yes d$Q$: no $\delta Q$: yes $\Delta Q$: no In the second bullet in the screenshot below

105 questions with answers in STATISTICAL PHYSICS

Venusian Mysteries – Part Two
Conservation of Energy Brilliant Math & Science Wiki

Introduction (to the Thematic Meeting 1.3 Science in Response to Basic Human Needs)

Open-Source Machine Learning in Computational Chemistry
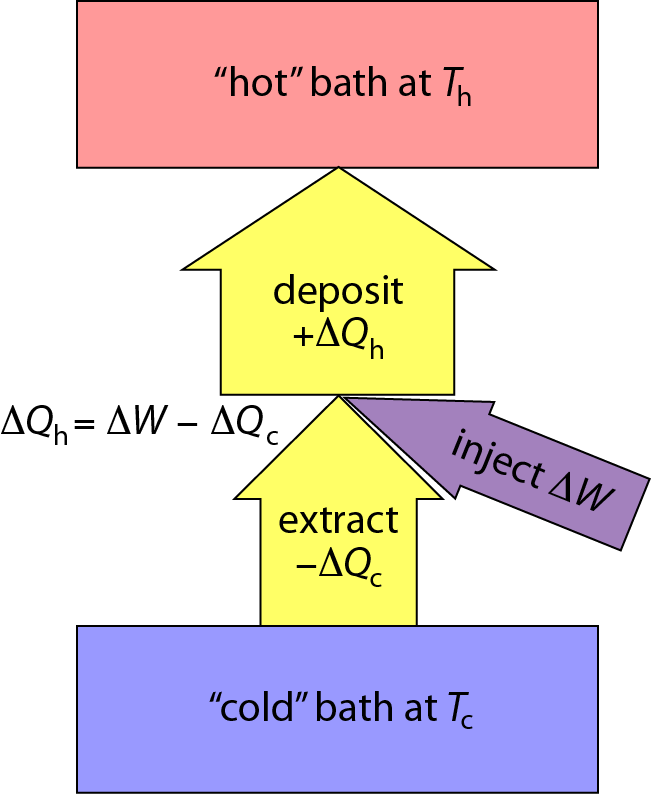
Heat Pumps Work Miracles

Casimir effect - Wikipedia
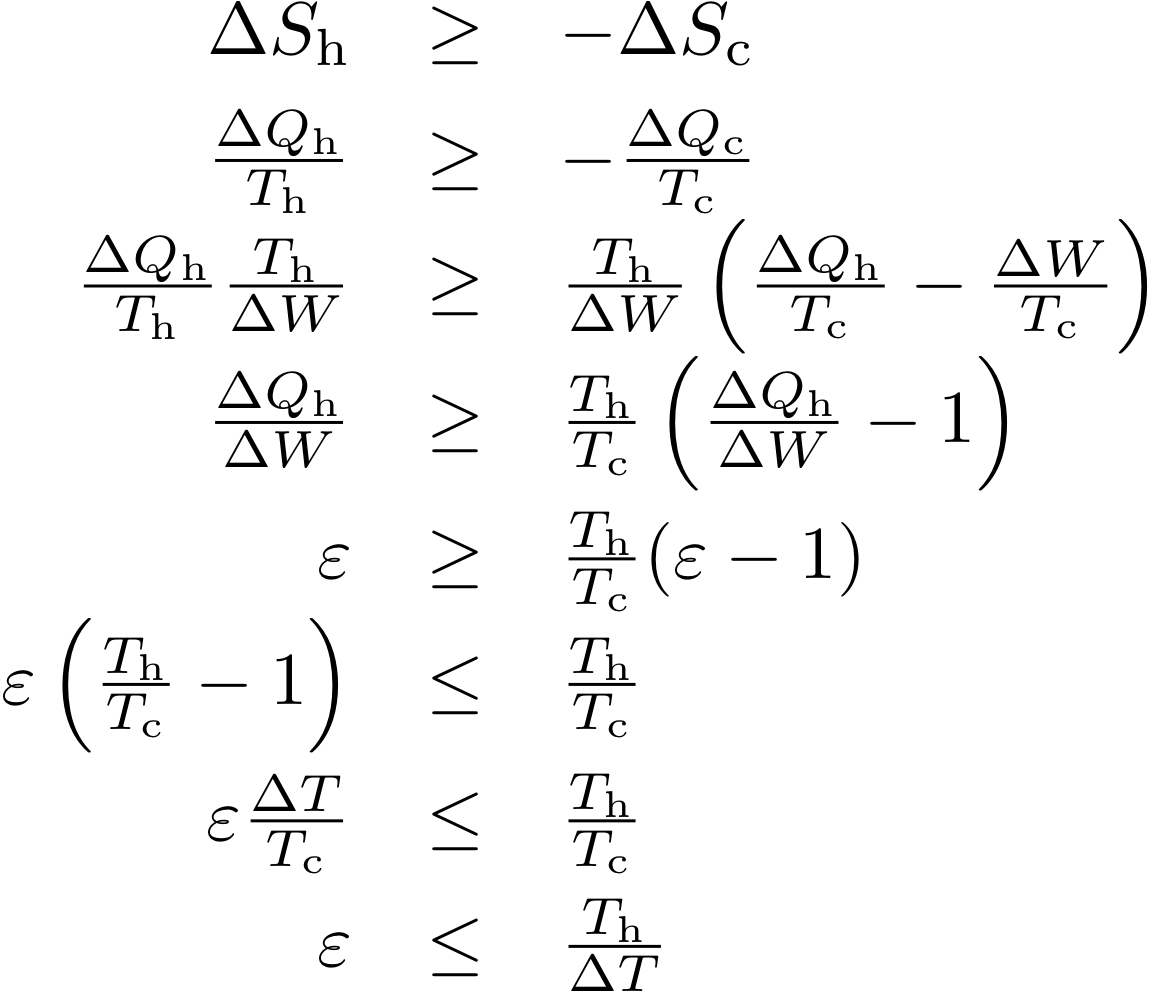
Heat Pumps Work Miracles

Can the Second law of thermodynamics be abandoned?

Apparent contradiction between Carnot's theorem and efficiency calculations!?
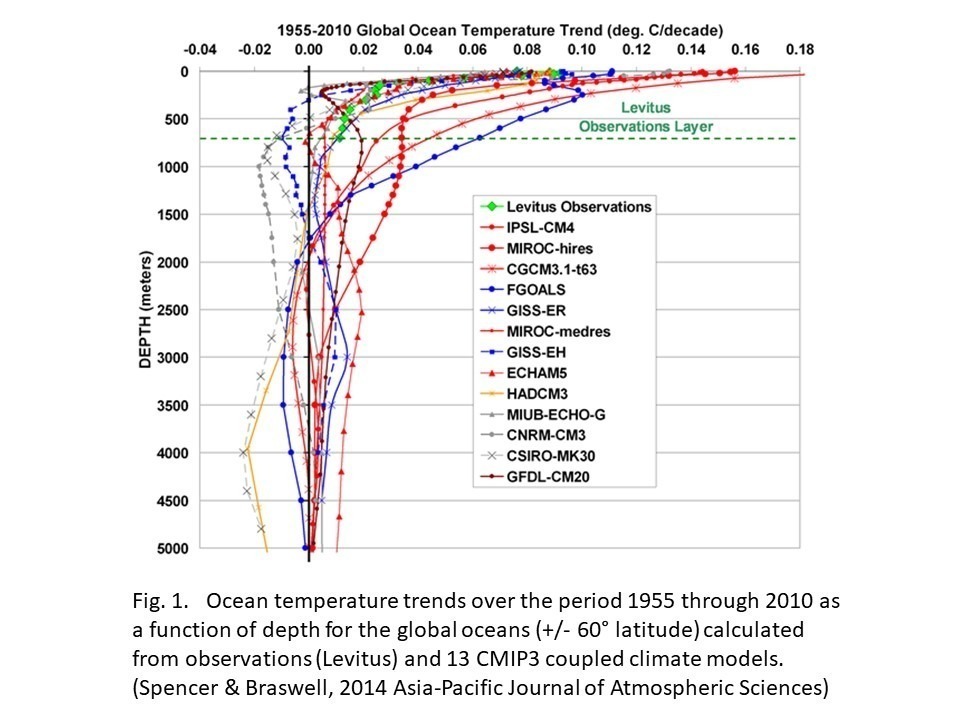
SITYS: Climate models do not conserve mass or energy
Sugerir búsquedas
También te puede interesar

Hicarer 2 péndulos de cristal de péndulo de chakras, péndulo de amatista natural, 7 péndulos, orgonita de adivinación de cristal piramidal, unisex

Funda de Cuero Cartera con Soporte Carcasa QH2 para Huawei Honor Magic6 Lite 5G Verde

Altavoces Gaming 5.1 ARENA 9 con Iluminación Reactiva

Hero9 Black update page
€ 24.50EUR
puntaje 4.5(179)
En stock
Continuar reservando
También te puede interesar

Hicarer 2 péndulos de cristal de péndulo de chakras, péndulo de amatista natural, 7 péndulos, orgonita de adivinación de cristal piramidal, unisex

Funda de Cuero Cartera con Soporte Carcasa QH2 para Huawei Honor Magic6 Lite 5G Verde

Altavoces Gaming 5.1 ARENA 9 con Iluminación Reactiva

Hero9 Black update page
€ 24.50EUR
puntaje 4.5(179)
En stock
Continuar reservando
©2018-2024, juliabrookeracing.com, Inc. o sus afiliados



